Tumor Heterogeneity
Lecture, Medizinische Fakultät, Christian-Albrechts-Universität zu Kiel, 2024
This lecture provides a brief introduction to the topic of tumor heterogeneity from the perspective of a mathematical oncologist.
Learing goals: to be able to distinguish forms of tumor heterogeneity, understand that they are a direct result of the complex process of cancer evolution, and to quantify heterogeneity from sequencing or cell classification (labeling) data using at least two different approaches.
You can find the slides (in German) here.
What are the origins and importance of tumor heterogeneity?
Tumor heterogeneity can result from diversification during the complex process of cancer evolution
Diversification can result from genetic and epigenetic mutations and genetic instability
Tumor heterogeneity is believed to be responsible for the evolution of both de novo and preexisting resistance to treatment
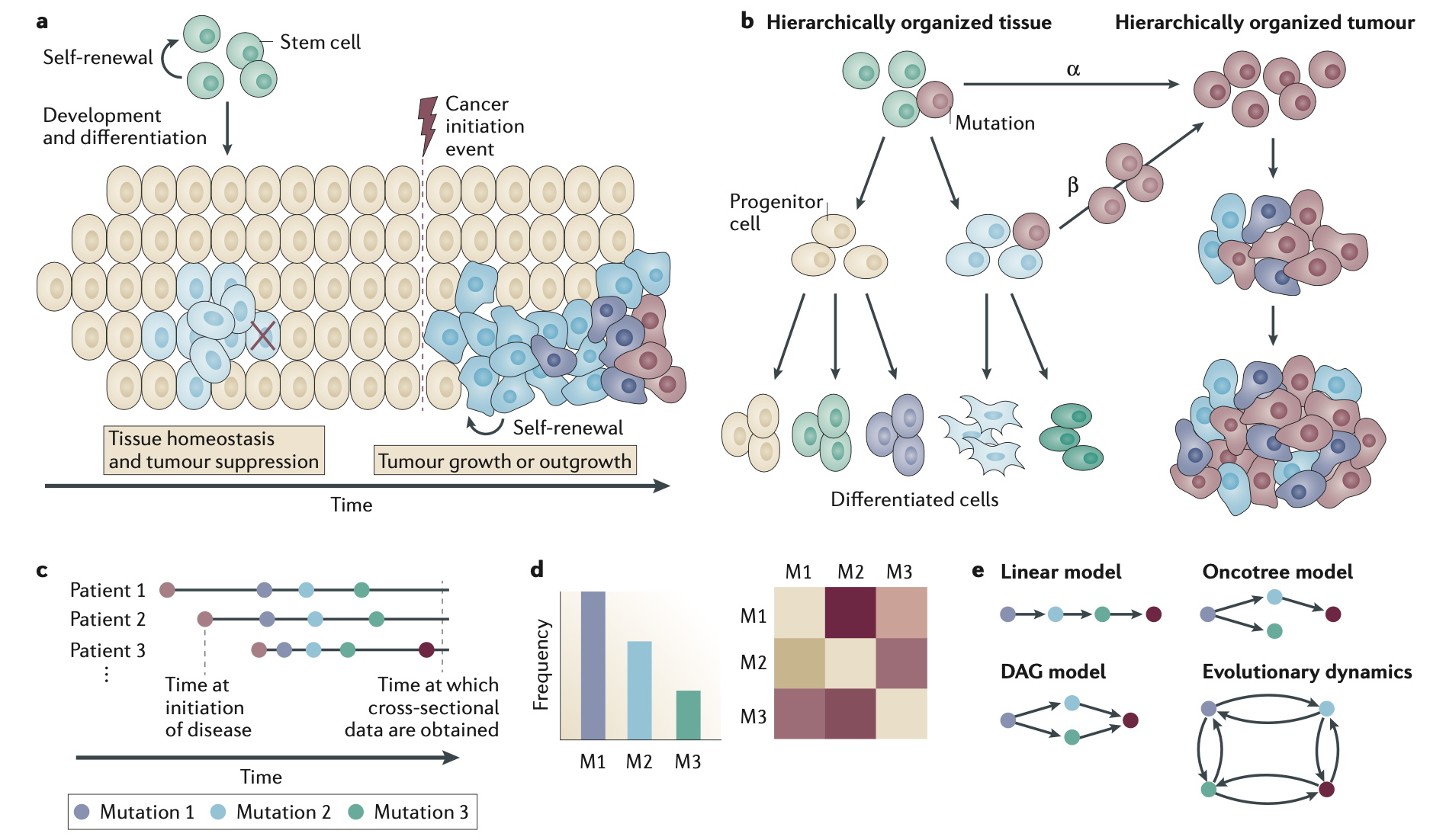
Tumor initiation and progression, from PMID: 26597528
Levels of tumor heterogeneity
Interpatient heterogeneity: Cancer cells of different patients can vary dramatically
Intratumor heterogeneity: Cancer cells of the same patient’s tumor can also vary dramatically
Intertumor heterogeneity: Cancer cells of different sites within one patient, e.g., from primary tumor and metastasis, can be different, but some metastases can be much more homogeneous again, see PMID: 32451459
To understand tumor heterogeneity, we need to understand cancer evolution
Cancer arises in the process of somatic evolution, that is, through spontaneous diversification and selection in cell populations of the human body.
A reiteraive process of expansion, diversificaiton, selection can take place.
By the process of clonal evolution, tumors harvest heterogeneity, depending on the mode of selection (e.g., neutral evolution, clonal interference, punctuated equilibrium, spatially isolated selection events). See this review
The role of plasticity
There is also evidence of diversification of cellular behavior, called plasticity
The increasing complexity of a tumor, e.g., driven by disorganized vasculature and conflicting signals to the immune system, opens the door for cellular plasticity programs to persist and for the coexistence of cell phenotypes
Phenotypic plasticity can result in niches within which cancer cells may better survive therapy and evolve long-term resistance
Plasticity does not only affect cancer cells, but also the cancer infiltrating stroma and immmune cells, e.g., fibroblasts and T lymphocytes.
Cancer also may inherit some of the tissue’s levels of differentiation, e.g., in the form of cancer stem cells (CSCs), which are uniquely capable of recapitulating a tumor and give rise to multiple lineages that can contribute in phenotypic tumor heterogeneity.
Tumor heterogeneity contributes to resistance to therapy
Radiotherapy: non-dividing persister (cancer stem) cells may not be affected as much
Chemotherapy: Genetic mutations and phenotypic plasticity can lead to low responses in cancer subpopulations
Targeted therapy: Persister cells can lead to de novo resistance; low-frequency pre-existing resistance is eventually selected (and may become more aggressive on the way)
Immune therapy: Immune-evading pathways are enriched during treatment; spatial constraints may hinder immune cell killing
Measuring tumor heterogeneity
Diversity index measures, such as Shannon index or related quantities, see generalized diversity
For example, using the Shannon index, Almendro et al. found that intratumor genetic diversity was tumor-subtype specific in breast cancers (comparing Luminal A/B, Triple negative, and Her2+ cancers). Yet, diversity did not change during treatment in tumors with partial or no response.
Diversity can also be assessed using median differences of allele frequencies, e.g., using “Mutant Allele Tumor Heterogeneity” (MATH), see PMID: 26840267
Literature
Marusyk A, Polyak K. Tumor heterogeneity: causes and consequences. Biochim Biophys Acta. 2010. PMID: 19931353
Meacham CE, Morrison SJ. Tumour heterogeneity and cancer cell plasticity. Nature. 2013. PMID: 24048065
Tabassum DP, Polyak K. Tumorigenesis: it takes a village. Nat Rev Cancer. 2015. PMID: 26156638
Morris LG et al. Pan-cancer analysis of intratumor heterogeneity as a prognostic determinant of survival. 2016. PMID: 26840267
Grzywa TM, Paskal W, Włodarski PK. Intratumor and Intertumor Heterogeneity in Melanoma. Transl Oncol. 2017. PMID: 29078205
Dagogo-Jack I, Shaw AT. Tumour heterogeneity and resistance to cancer therapies. Nat Rev Clin Oncol. 2018. PMID: 29115304
